In elderly patients, severe symptomatic aortic stenosis is often caused by the build-up of calcium (mineral deposits) on the aortic valve’s leaflets (flaps of tissue that open and close to regulate the one-way flow of blood through the aortic valve). This build-up of calcium on the leaflets impairs the aortic valve’s ability to fully open and close. As a result, the narrowed valve allows less oxygen-rich blood to flow from the lungs to the brain and rest of the body which may cause symptoms like severe shortness of breath and extreme fatigue.
Severe Aortic Stenosis
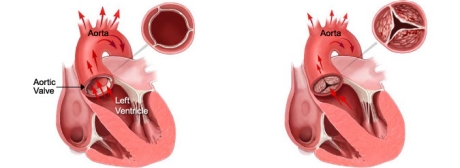
Fig. 1 depicts the leaflets of a healthy aortic heart valve which open wide to allow oxygen-rich blood to flow unobstructed in one direction. The blood flows through the valve into the aorta where it then flows out to the brain and rest of the body.
Fig. 2 depicts the leaflets of a stenotic or calcified aortic valve unable to open wide, obstructing blood flow from the left ventricle into the aorta.
Treatment
While open-chest surgery to replace the aortic valve is the gold standard treatment for severe symptomatic aortic stenosis, there are some patients who are not eligible for surgery or considered at high risk for surgery. These patients may be candidates for a therapy called transcatheter aortic valve replacement (TAVR), which allows Heart Teams to replace a diseased aortic heart valve without open-chest surgery. TAVR enables the placement of a balloon-expandable heart valve into the body with a tube-based delivery system called a catheter. This tube-based system allows the valve to be inserted through an incision in the leg and into an artery (transfemoral procedure), or through an incision between the ribs and then through the bottom end of the heart called the apex (transapical procedure). The transapical procedure is only available to certain high-risk patients who are not candidates for the transfemoral procedure because they do not have appropriate access through their leg artery.
A Heart Team will conduct a comprehensive evaluation to determine whether the TAVR procedure is an appropriate therapeutic option. In certain cases, TAVR may not be an option because of co-existing medical conditions or disease processes that would prevent the patient from experiencing the expected treatment benefit or because the risks outweigh the benefits. For those who are candidates for TAVR, this therapy may provide relief from the often debilitating symptoms associated with severe symptomatic native aortic valve stenosis. TAVR is a significant procedure involving general anesthesia, and placement of the valve is associated with specific contraindications as well as serious adverse effects, including risks of death, stroke, damage to the artery used for insertion of the valve, major bleeding, and other life-threatening and serious events. In addition, the longevity of the valve’s function is not yet known. Please refer to the Instructions for Use or Patient Brochure for complete information on warnings, contraindications and serious adverse events.